Greetings, budding researchers! Are you ready to explore the realm of atoms as well as the GCSE atoms periodic table? We are going to take a trip inside the matter and the things that it is made out of, and how these little bits frame such a huge range of different elements that we have around us.
Think of every object and material that you see or even interact with as being made up of very small, these particles which are called atoms. These atoms are so small that it is impossible to even see a million of them arranged one after the other perpendicular to your line of sight. But do not be deceived by their smallness –They are the very basis of all things in existence, from a simple pencil to that enormous star hanging in the sky.
At the center of an atom there is nucleus, containing neutrons (they are neutral, giving stability and balance) and protons (positively charged and mass similar to neutrons)
Electrons remain at discrete energy states, commonly known as shells, in the region of nucleus, every shell containing a specific number of electrons. Every shell is consists of subshells, and electrons occupy these subshells according to a particular order referred to as the Aufbau principle. This configuration of electrons explains the properties which an atom exhibits.
Identical element’s atoms can vary in number of neutrons, forming isotopes. Isotopes have similar number of protons but contrast in mass due to differences in number of neutrons, such as carbon having either or 8 neutrons.
Alkali metals such as lithium (Li) and sodium (Na), both are very reactive as it is easy to remove its only electron from them. When more groups are traversed, additional electron shells tend to shear the valence electrons from the nucleus, thus making the element more reactive.
The atomic number of an element accelerates as you move in a horizontal row from left to right. This means that the atomic nucleus exerts a greater attractive force on the electrons surrounding it. Consequently, the tendency of the elements to exhibit metallic features (such as lustrousness) decreases, while the tendency to react with the elements located on the right side of the periodic table, increases.
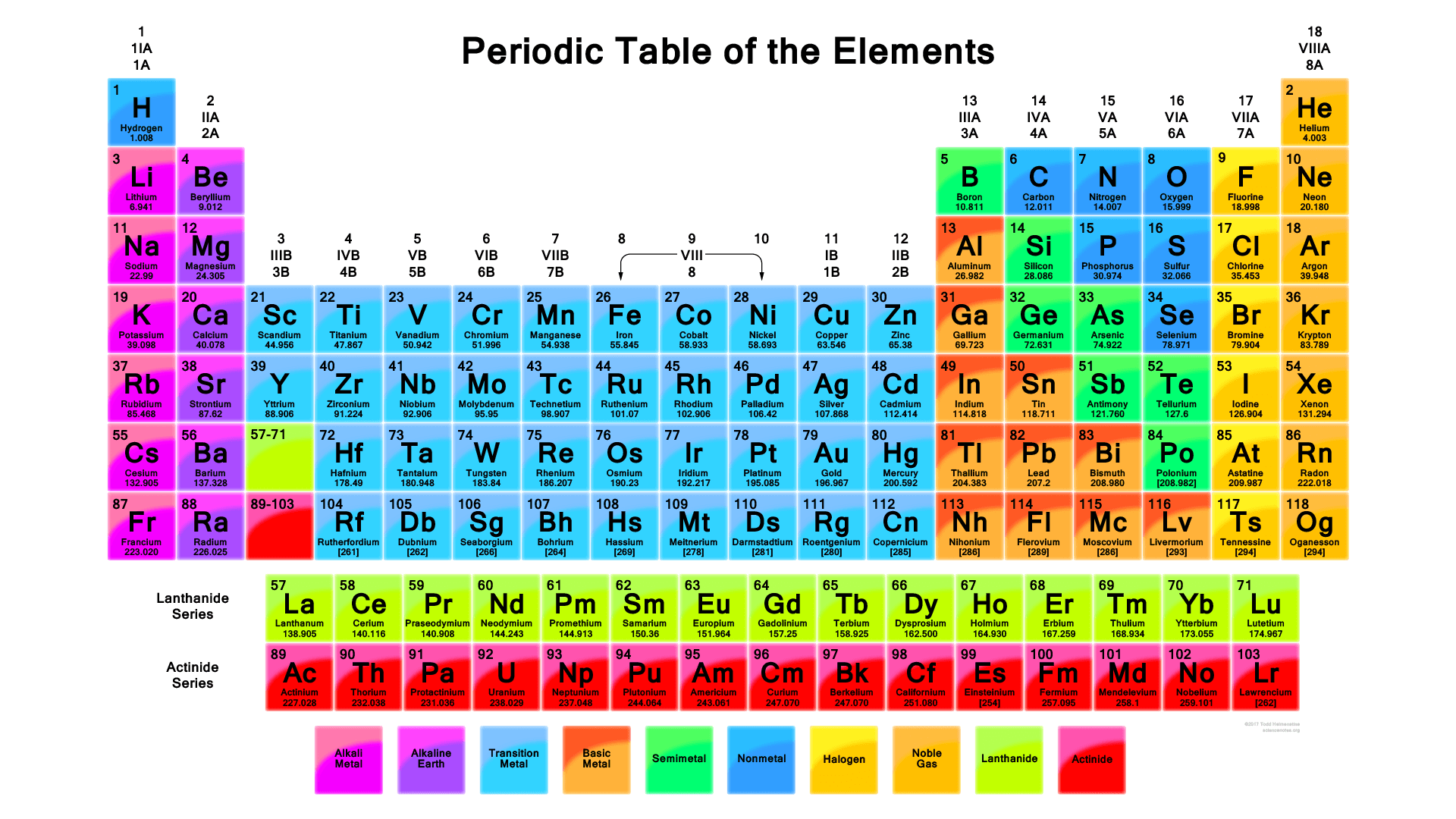
Let’s delve deeper into the secrets of the GCSE atoms periodic table and how it helps us understand the elements and their interactions.
The outermost layer of an atom is known as the valence shell. Any chemical bonding that occurs in the atom takes place with the valence electrons. They are considered to be the “matchmakers” who help in the bonding of the atoms. Metals which are electropositive in nature tend to give up their electrons and form cations which attach themselves to anions as in the case of sodium chloride (NaCl). Non-metals on the other hand share electrons that are used to form bonds and in this case it is a covalent bond as in methane(CH₄) and no atom is left unstable.
Here’s a comparison between metals and non-metals:
| Property | Metals | Non-metals |
|---|---|---|
| Physical State (at room temperature) | Mostly solids | Solids, liquids, or gases |
| Appearance | Shiny | Dull |
| Electrical Conductivity | Good conductors | Poor conductors |
| Thermal Conductivity | Good conductors | Poor conductors |
| Malleability (can be hammered into thin sheets) | Yes | No (brittle) |
| Ductility (can be drawn into thin wires) | Yes | No |
The periodic table is an excellent means of predicting elements properties depending on their location. Below are some of the trends to note:
Metallic Character: In a group (column), as one travels downwards, there is an increase in the metallic character. In other words, elements have better conductivity, enhanced malleability, and lose electrons more readily.
Non-Metallic Character: In a period (row), unlike in the previous trend, the degree of nonmetallic property diminishes from left to right. The elements become less good conductors, more brittle, and prefer to gain electrons.
Electronegativity: It usually becomes more pronounced when going up a group and from the left to the right of a period. Non-metals are typically those elements that are highly electronegative, that is, good electron acceptors, and metals are those elements that have low electronegativity and can easily give away electrons.
Here are some example questions based on the concepts we covered and how you can solve them using your knowledge:
Question 1 (2021, OCR GCSE Combined Science Trilogy Paper 2)
Explain why chlorine (Cl) is in the same group (column) of the periodic table as fluorine (F).
Solution:
Both chlorine and fluorine belong to Group 17 (halogens). Both chlorine and fluorine have 7 valence electrons in their outermost shell. This configuration makes them highly reactive as they readily gain one electron to achieve a stable octet configuration.
Remember: These are just examples, and the difficulty level of questions can vary depending on the specific exam board and year.
Strategies for doing previous year question papers:
Read questions attentively: Find the important definitions and what is exactly been asked in question.
Use your wisdom of periodic table and atoms: Apply the topics that have been discussed, such as ionic, covalent bonding, periodic trends and valence electrons to solve question.
Define your Understanding: Demonstrate your comprehension and calculations to exhibit your knowledge.
Have a look at marking scheme: After you have solved the question, you must check out marking scheme it is easily accessible through the official website of the board as this will help you to know how the marks are given and make sure you have studied every aspect.
By preparing with previous year paper and using your understanding of periodic table and atom, you will be fully ready to handle GCSE Chemistry exams with certainty.
What is an atom?
An atom is the smallest unit of matter that can participate in a chemical reaction. It consists of a central nucleus containing protons and neutrons, surrounded by electrons.
What are protons, neutrons, and electrons?
Protons: Positively charged particles in the nucleus, determining the element’s identity (atomic number).
Neutrons: Particles with no electrical charge in the nucleus, adding mass to the atom.
Electrons: Negatively charged particles orbiting the nucleus, involved in bonding with other atoms.
What are electron shells and orbitals?
Electron shells are energy levels around the nucleus where electrons reside. Orbitals are specific pathways within a shell where electrons can exist.
What is the periodic table?
The periodic table is a chart that organizes all known elements based on their atomic number, electron configuration, and recurring properties.
What is the difference between metals and non-metals?
Metals are generally shiny, good conductors, and lose electrons to form cations (positive ions). Non-metals are often dull, poor conductors, and gain electrons to form anions (negative ions).
What are ionic and covalent bonds?
Ionic bonding: Electrostatic attraction between oppositely charged ions (metal loses electron, non-metal gains electron). Example: Sodium chloride (NaCl).
Covalent bonding: Sharing of electrons between atoms to achieve a stable configuration (full valence shell). Example: Methane (CH₄).
What is the octet rule?
An atom is generally most stable when its outermost shell has 8 electrons. This drives electron gain/loss and bonding behavior.
How can the periodic table be used to predict properties?
Periodic trends like increasing metallic character down a group or increasing electronegativity across a period allow prediction of element properties based on their position.
Chemistry Bench is 100% online chemistry tutoring platform that provides quality education for various chemistry courses and curricula on the national and international levels.
