Atomic size is one of the most important factors in chemistry, where we seek to explain the types of substances, their forms, and changes in the interaction of different substances. This document focuses on commonly asked questions in the option of NEET Chemistry, others like astronomy, the structural concept of elements in molecules, the nature of electromagnetic radiation, and the photoelectric effect.
Electromagnetic radiation is a form of energy that travels through space as oscillating electric and magnetic fields. This radiation spans a broad spectrum, from gamma rays to radio waves, with visible light falling in between. Understanding electromagnetic radiation is crucial for grasping how atoms interact with light and other forms of energy.
The electromagnetic spectrum encompasses all types of electromagnetic radiation. Here’s a brief overview of its regions:
The photoelectric effect is the phenomenon where light incident on a material ejects electrons from its surface. This effect provided critical evidence for the quantum nature of light, leading to the development of quantum mechanics.
Albert Einstein explained the photoelectric effect by proposing that light consists of packets of energy called photons. The energy of a photon is given by:
E=hν
where h is Planck’s constant (6.626×10^−34 Js) and ν is the frequency of the light.
For an electron to be ejected from a material, the energy of the incoming photon must be greater than the work function (ϕ) of the material. The kinetic energy (KE) of the ejected electron is then:
KE=hν−ϕ
The hydrogen atom, with its single electron, provides a simple yet profound system for studying atomic spectra. When hydrogen gas is excited by an electric discharge, it emits light at specific wavelengths, forming a line spectrum.
Johann Balmer empirically derived an equation to predict the wavelengths of visible lines in the hydrogen spectrum. This series, known as the Balmer series, is given by:
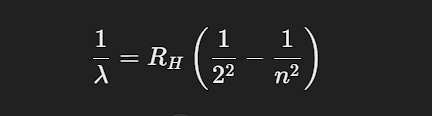
where RH is the Rydberg constant (1.097×10^7 /m) and n is an integer greater than 2.
Niels Bohr’s model of the hydrogen atom was a groundbreaking advancement in atomic theory. It introduced the idea of quantized electron orbits and explained the stability of atoms and their emission spectra.
Quantized Orbits: Electrons orbit the nucleus in fixed energy levels without radiating energy.
Angular Momentum Quantization: The angular momentum of an electron in orbit is quantized and given by:
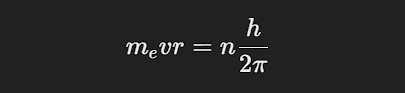
where me is the electron’s mass, v is its velocity, r is the orbit radius, h is Planck’s constant, and n is a positive integer (principal quantum number).
Energy Levels: The energy of an electron in a particular orbit is given by:
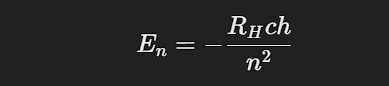
where RH is the Rydberg constant for hydrogen.
Radiative Transitions: Electrons can transition between orbits by absorbing or emitting photons of specific energies, corresponding to the difference in energy levels:
where Ef and Ei are the final and initial energy levels, respectively.
The energy of the electron in the nnn-th orbit is derived from Coulomb’s law of electrostatic attraction between the positively charged nucleus and the negatively charged electron, balanced by the centripetal force of the orbiting electron.
For the n-th orbit:
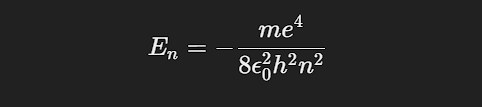
where e is the electron charge, ϵ0 is the permittivity of free space, and h is Planck’s constant.
The radius of the n-th orbit is given by:
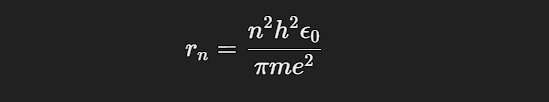
While the Bohr model was revolutionary, it had several limitations:
The study of atomic structure, encompassing the nature of electromagnetic radiation, the photoelectric effect, and the Bohr model of the hydrogen atom, lays the groundwork for understanding the behavior of atoms and molecules. These fundamental concepts highlight the interplay between energy and matter, forming the basis for modern chemistry and physics. By grasping these principles, we unlock the mysteries of the atomic world, paving the way for advancements in science and technology.
For those interested in delving deeper into atomic structure and related phenomena, the following resources are recommended:
Understanding atomic structure is not just about memorizing equations and concepts; it’s about appreciating the profound implications these have on the nature of reality itself. Happy studying!
Foundation of Chemistry: Atomic structure is the fundamental concept upon which the entire subject of chemistry is built. A solid understanding of atomic structure is essential for mastering other chemistry topics in the NEET syllabus.
High Weightage in Exams: Questions related to atomic structure frequently appear in NEET exams. Topics like Bohr’s model, the photoelectric effect, and electromagnetic radiation are regularly tested, contributing significantly to your overall score.
Conceptual Clarity: A deep understanding of atomic structure concepts enhances your ability to solve complex problems and understand advanced topics in physical chemistry, organic chemistry, and inorganic chemistry.
Interdisciplinary Relevance: Concepts from atomic structure overlap with physics, especially in areas like quantum mechanics and electromagnetic waves. This interdisciplinary knowledge can help you tackle questions in both chemistry and physics sections.
Application-Based Questions: NEET exams often include application-based questions. Knowing the practical implications of atomic structure, such as the photoelectric effect and spectral lines, can help you solve such questions more effectively.
Problem-Solving Skills: Understanding the derivations and mathematical formulations in atomic structure topics enhances your problem-solving skills, which is crucial for scoring well in NEET.
Critical Thinking: Grasping the limitations of models like Bohr’s encourages critical thinking and helps you understand the evolution of scientific theories, a skill that’s beneficial for comprehending other complex scientific concepts.
Experimental Questions: NEET exams include questions based on experimental observations and results. Knowledge of atomic spectra and the photoelectric effect is essential for interpreting experimental data and answering such questions accurately.
Preparation for Higher Studies: Mastering atomic structure not only helps in NEET but also lays a strong foundation for future studies in medical and dental courses, where a thorough understanding of basic sciences is crucial.
Boosts Confidence: A solid grasp of atomic structure topics boosts your confidence, making you better prepared to face the NEET exam. Confidence in fundamental topics translates to overall better performance in the exam.
1. Why is it necessary to comprehend electromagnetic radiation for NEET?
Understanding electromagnetic radiation makes it easier to understand processes such as the photoelectric effect and atomic emission spectra, which are often covered in NEET.
2. Why can the photoelectric effect be an important topic in the NEET chemistry course?
The photoelectric effect shows that light has a particle aspect, making students apply the concepts of energy quantization and motion of electrons both in chemistry and physics, and therefore it can be included in NEET.
3. Does the Bohr model come in the NEET exam?
Bohr’s model is important in explaining the atomic structure and also the spectral lines. Thus, questions concerning the energy levels and radius of orbits of an electron as given by this model in most NEET examinations are common.
4. How will knowledge of atomic spectra be useful in NEET preparations?
Knowledge of atomic spectra assists in the understanding of the emission and absorption spectra, which assists in the identification of elements, a common topic in NEET.
4. Why should we focus on the Bohr’s model’s flaws?
Understanding the limitations of Bohr’s model encourages a more comprehensive approach to atomic concepts, which will be useful for theory as well as application-based questions in NEET.
In other words, the energy that is released and possessed as alternating electric and magnetic fields that propagate in empty space is known as electromagnetic radiation. This kind of energy radiation is massive, differing from X-rays, UV rays, visible rays, gamma rays, infrared rays and microwave ranges to the radio frequency range.
One typical definition of the effect is the ejection of electrons from a metal plate when light strikes it. This phenomenon was a catalyst for the development of quantum physics.
Explanation by Einstein of the Photoelectric Effect: According to Einstein the photoelectric effect is the light that has particles called photons, which are considered to be energy vectors of light. The energy of every photon is described as follows:
E=hν
The kinetic energy of the ejected electron is defined as:
KE=hν−ϕ
The hydrogen atom, with its single electron, serves as a simple model for studying atomic spectra.
Balmer Series: It is expressed as:
Bohr’s model proposed the idea of quantized orbits, thereby explaining the atomic stability and atomic spectra.
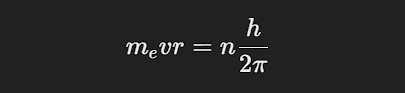
Quantized Orbits: At set energy levels, electrons revolve around the nucleus.
Angular Momentum Quantization: It is given by:
Energy Levels: The electron’s energy in a particular orbit is:
Radiative Transitions: Electrons move between orbits by absorbing or emitting photons with energy equal to the difference between initial and final energy levels.
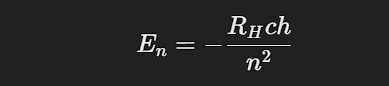
Multi-Electron Atoms: It cannot accurately predict spectra for atoms with more than one electron.
Zeeman Effect: It does not explain spectral line splitting in magnetic fields.
Wave-Particle Duality: It overlooks the wave nature of electrons, later addressed by quantum mechanics.
Limited Accuracy: The model’s predictions are precise only for hydrogen-like atoms.
Conceptual Clarity: Furthermore, revision of the theory of the atom is beneficial in solving even the most complex issues in physical and organic, as well as inorganic chemistry.
Interdisciplinary Relevance: Parts of chemistry such as atomic structure, quantum theory, and propagation of particles in waves have indivisible relations with the area of physics.
Improvement of skills: Understanding the concepts of atomic structure, involving mathematical formulations and derivations, reinforces problem-solving abilities and that is important for achieving high score in NEET.
New approach Questions: NEET involves questions on data interpretation and observations of experiments which depends on photoelectric effect and knowledge of atomic spectra.
Preparation for Higher Studies: Mastering atomic structure aids NEET success and builds a foundation for medical and dental courses where strong basic science knowledge is critical.
Readiness for Further Studies: Command over atomic structure not only helps clear NEET but also prepares the ground for medical and dental studies where a good command of the basic sciences is necessary.
Enhances self-esteem: A firm understanding of atomic structure enhances self-esteem and readiness to sit for examinations.
For those interested in going deeper into atomic structure and related phenomena, the following resources are recommended:
Happy studying!
1. Understanding of electromagnetic radiation is considered important in Neet. what is the reason behind that?
Learning electromagnetic radiation helps in understanding the processes like the atomic emission spectra and photoelectric effect, which are frequently covered in NEET.
2. Why photoelectric effect can be a crucial topic in the chemistry course of NEET?
The photoelectric effect shows that light has a particle aspect, making students apply the concepts of energy quantization and motion of electrons both in chemistry and physics, and therefore it can be included in NEET.
3. Does the Bohr model come in the NEET exam?
Bohr’s model is important in explaining the atomic structure and also the spectral lines. Thus, questions concerning the energy levels and radius of orbits of an electron as given by this model in most NEET examinations are common.
4. How will knowledge of atomic spectra be useful in NEET preparations?
Knowledge of atomic spectra assists in the understanding of the emission and absorption spectra, which assists in the identification of elements, a common topic in NEET.
Chemistry Bench is 100% online chemistry tutoring platform that provides quality education for various chemistry courses and curricula on the national and international levels.
