Welcome, young scientists, to the fascinating world of atoms and the periodic table! Today, we’ll embark on a journey to understand the tiniest components of matter and how they organize themselves into the incredible variety of elements we see around us.
Imagine everything you see and touch is built from incredibly tiny particles called atoms. These atoms are so small you wouldn’t be able to see even a million of them lined up, side-by-side, with your naked eye! But don’t let their size fool you – they’re the fundamental building blocks of everything in the universe, from your pencil to the giant star in the sky.
Think of an atom like a miniature solar system. At its center, we have the nucleus, a very dense region containing two types of subatomic particles:
Around the nucleus, whizzing like tiny planets, are even smaller particles called electrons. These have a negative electrical charge and a much smaller mass compared to protons and neutrons. The number of electrons in an atom usually equals the number of protons, making the atom electrically neutral.
Electrons don’t just zoom around randomly. They occupy specific energy levels called electron shells. Imagine these shells like concentric rings around the nucleus. The closer a shell is to the nucleus, the lower its energy level. Each shell can hold a certain number of electrons. The first shell, closest to the nucleus, can only hold 2 electrons. The second shell can hold up to 8 electrons, and so on.
But it gets a little more interesting! Within each shell are even smaller regions called orbitals. Think of them as specific pathways electrons can take within a shell. Some shells have only one orbital, while others have multiple. Electrons fill orbitals in a specific order, following a principle called the aufbau principle. This arrangement of electrons in different shells is called the electronic configuration of an atom and plays a crucial role in how elements behave.
Not all atoms of the same element are exactly alike! Sometimes, they can have different numbers of neutrons. These variations are called isotopes. While they have the same number of protons (and hence the same element), the different number of neutrons gives them slightly different masses. For example, most carbon atoms have 6 protons and 6 neutrons, but there’s a rarer isotope with 6 protons and 8 neutrons!
Now, let’s talk about the amazing periodic table, a chart that organizes all the known elements. Elements are arranged in rows (periods) and columns (groups) based on their properties, particularly the arrangement of electrons in their outermost shell (valence electrons). This outermost shell is the most important for chemical reactions, as these electrons are involved in bonding with other atoms.
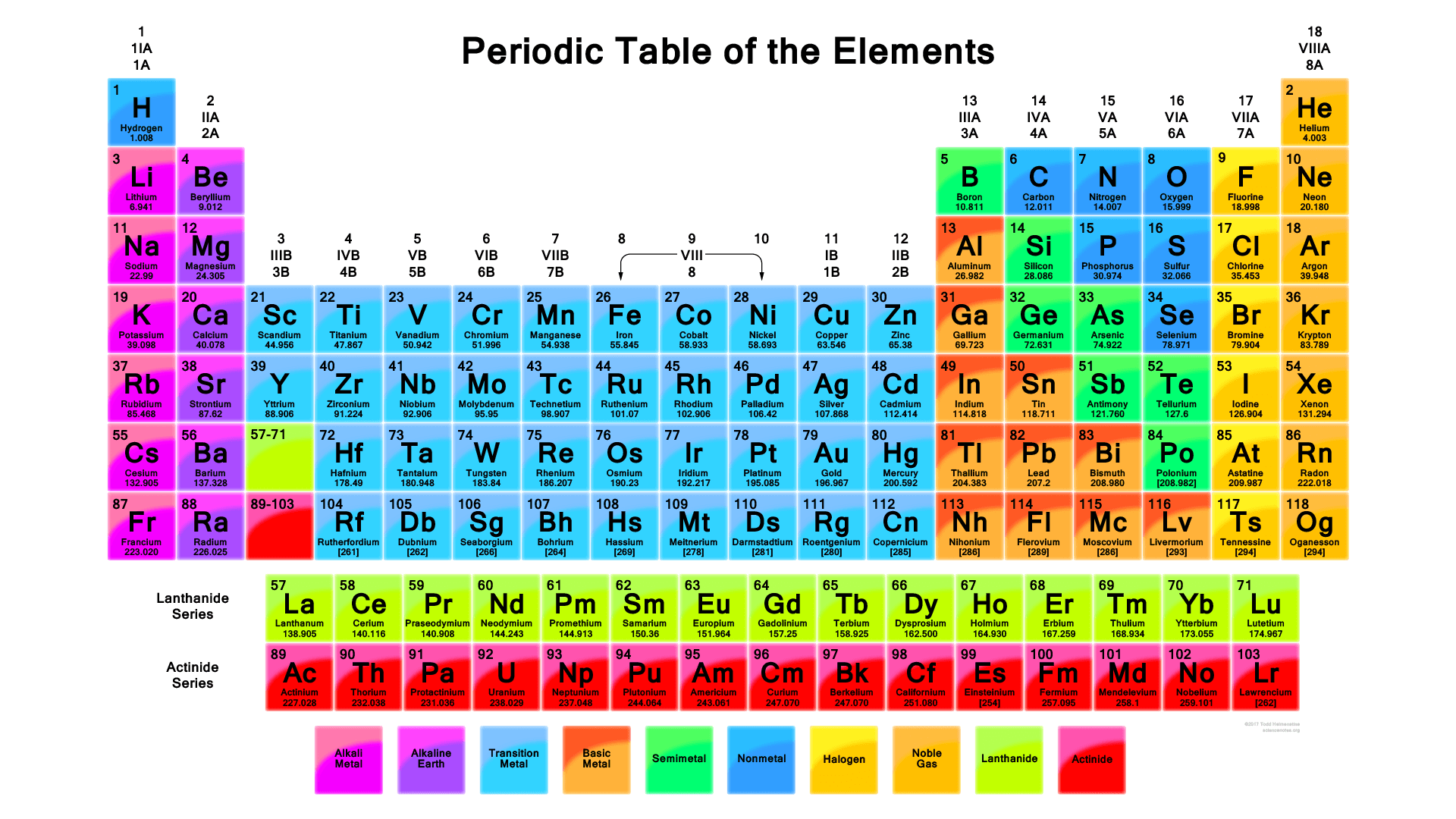
Elements in the same group (column) share similar properties because they have the same number of valence electrons. For example, Group 1 (alkali metals) like lithium (Li) and sodium (Na) all have one electron in their outermost shell. This makes them very reactive, meaning they readily lose that electron to form ionic bonds with other elements.
As you move down a group, the number of electron shells increases (remember periods tell you the number of shells). This means the valence electrons are further away from the nucleus and experience a weaker attraction. This makes them even more reactive!
Moving across a period (row) from left to right, the number of protons (and electrons) increases. This means the pull of the positively charged nucleus on the electrons gets stronger. As a result, the elements tend to become less metallic (lose their shiny properties) and more reactive towards other elements on the right side of the table.
We’ve explored the fascinating world of atoms and their building blocks. Now, let’s delve deeper into the secrets of the periodic table and how it helps us understand the elements and their interactions.
As mentioned earlier, the outermost shell of an atom, particularly the number of electrons it holds (valence electrons), plays a crucial role in how elements interact with each other. These valence electrons are like the “matchmakers” of chemistry! They determine an element’s tendency to form bonds with other elements.
The type of bonding an element participates in (ionic or covalent) significantly influences its physical and chemical properties. Here’s a comparison between metals and non-metals:
| Property | Metals | Non-metals |
|---|---|---|
| Physical State (at room temperature) | Mostly solids | Solids, liquids, or gases |
| Appearance | Shiny | Dull |
| Electrical Conductivity | Good conductors | Poor conductors |
| Thermal Conductivity | Good conductors | Poor conductors |
| Malleability (can be hammered into thin sheets) | Yes | No (brittle) |
| Ductility (can be drawn into thin wires) | Yes | No |
The periodic table is a powerful tool for predicting the properties of elements based on their position. Here are some key trends to remember:
Now that you’ve grasped the basics of atoms and the periodic table, try your hand at these questions:
Remember, the periodic table is a treasure trove of information. As you delve deeper into chemistry, you’ll discover even more fascinating patterns and relationships between elements. Keep exploring, keep questioning, and have fun on your scientific adventure!
Question 1 (2022, AQA GCSE Chemistry Paper 1)
State the number of protons, neutrons, and electrons in an atom of sodium-23 (²³Na).
Solution:
Question 2 (2021, OCR GCSE Combined Science Trilogy Paper 2)
Explain why chlorine (Cl) is in the same group (column) of the periodic table as fluorine (F).
Solution:
Both chlorine and fluorine belong to Group 17 (halogens). Elements in the same group share similar properties because they have the same number of valence electrons in their outermost shell. Both chlorine and fluorine have 7 valence electrons in their outermost shell. This configuration makes them highly reactive as they readily gain one electron to achieve a stable octet configuration.
Remember: These are just examples, and the difficulty level of questions can vary depending on the specific exam board and year.
Tips for solving past paper questions:
By practicing with past papers and applying your knowledge of atoms and the periodic table, you’ll be well-equipped to tackle GCSE Chemistry exams with confidence
What is an atom?
An atom is the smallest unit of matter that can participate in a chemical reaction. It consists of a central nucleus containing protons and neutrons, surrounded by electrons.
What are protons, neutrons, and electrons?
What are electron shells and orbitals?
Electron shells are energy levels around the nucleus where electrons reside. Orbitals are specific pathways within a shell where electrons can exist.
What is the periodic table?
The periodic table is a chart that organizes all known elements based on their atomic number, electron configuration, and recurring properties.
What are groups and periods in the periodic table?
What is the difference between metals and non-metals?
Metals are generally shiny, good conductors, and lose electrons to form cations (positive ions). Non-metals are often dull, poor conductors, and gain electrons to form anions (negative ions).
What are ionic and covalent bonds?
What is the octet rule?
An atom is generally most stable when its outermost shell has 8 electrons. This drives electron gain/loss and bonding behavior.
What is electronegativity?
A measure of an atom’s attraction for electrons in a bond. It influences bond type (ionic/covalent) and reactivity.
How can the periodic table be used to predict properties?
Periodic trends like increasing metallic character down a group or increasing electronegativity across a period allow prediction of element properties based on their position.